So You Want to Launch? – Key Considerations for Clinical-Stage Companies Before the “Big Day”
by Auxilius on Sep 18, 2023 6:09:02 AM
It is expected that nearly 67% of new biopharma product launches in the next 12 months will come from “first time launchers”. For clinical-stage biotech companies, representing the lifeblood of industry and patient-focused innovation, the leap into commercial launch is daunting. Below we outline some key activities that remain important with active clinical trials, as well as a suggested scale-up timeline to advance from clinical to commercial. With commercialization hanging in the balance, companies often must expand or reprioritize their accounting, finance and market access functions significantly.
Why Automation and Efficiency Matters
In lieu of hiring and incurring additional headcount, increasingly companies are looking to reduce the manual burden and workload in low return tasks and reprioritize existing talent/team members to revenue related roles. And, with new focus on commercial finance activities, public filers with ongoing pipeline and active clinical programs can’t afford a miss on the bottom – even if considered nonmaterial.
Clinical trial budget management, accruals and related financial reporting often represents the obvious opportunity for time savings, efficiency and risk reduction. Automation of clinical trial finance and accounting not only reduces spreadsheet error risk, but it frees up valuable talent to eventually lift and shift into commercial finance activities.
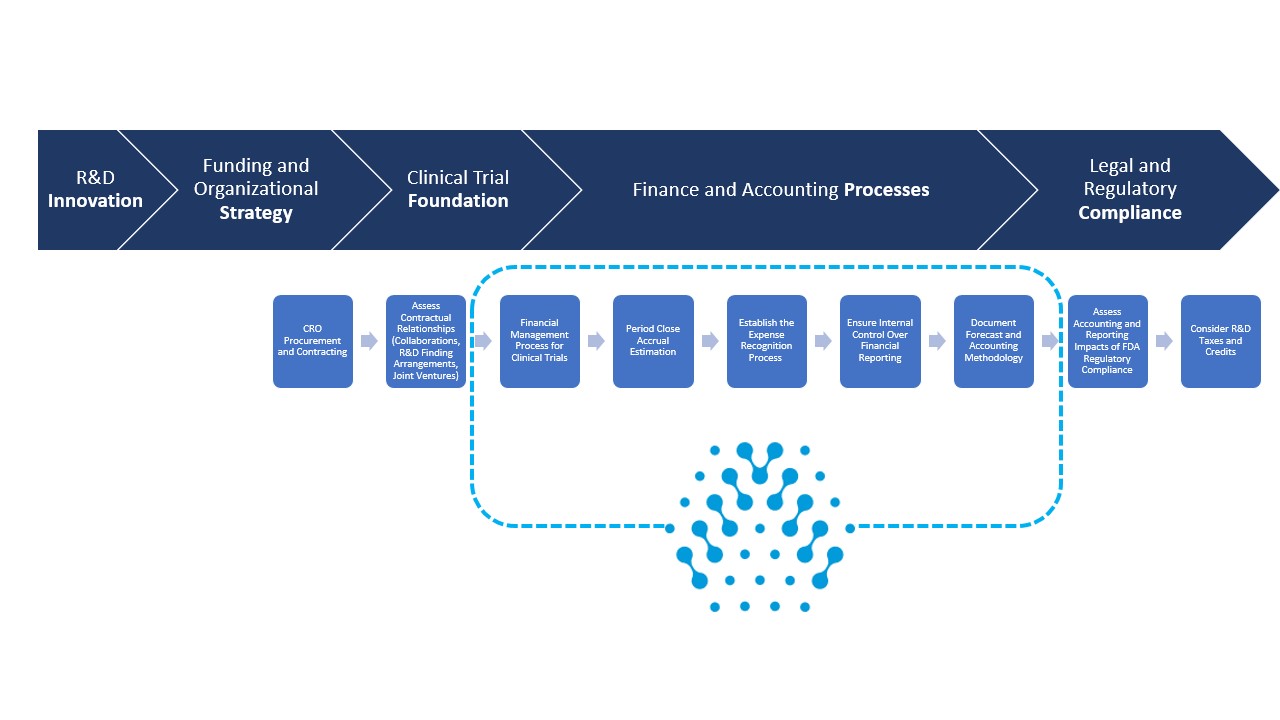
New Responsibilities for Finance and Accounting
The scale-up timeline for advancement to commercial revenue should start 18-24 months pre-NDA.
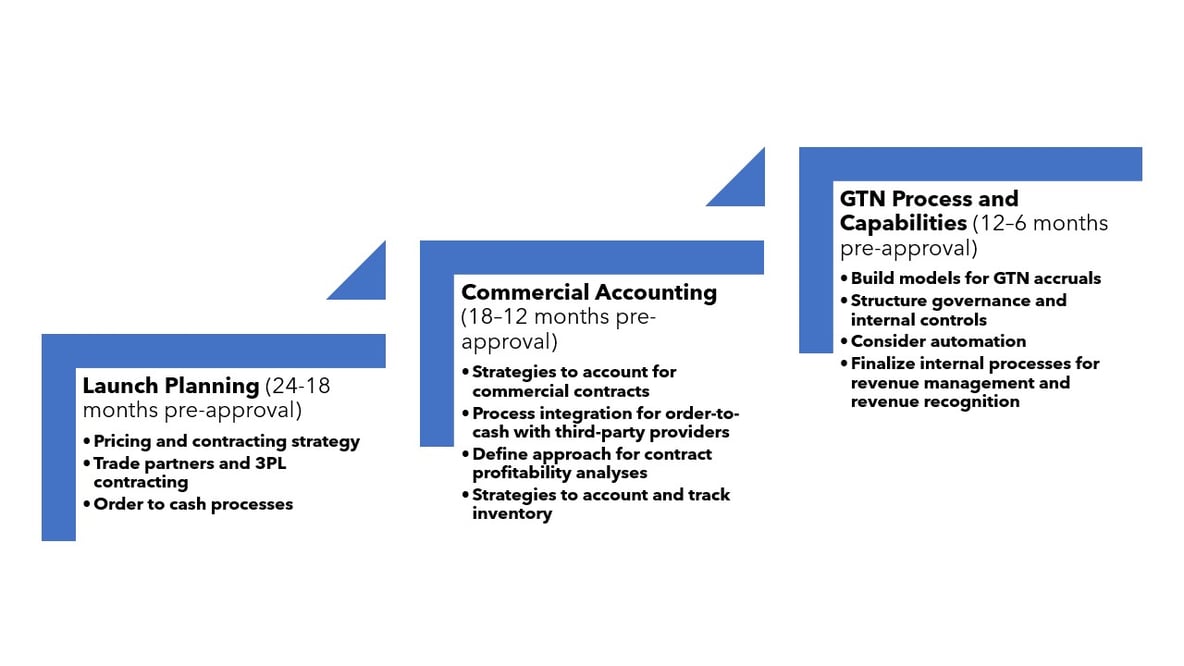
Consider these key steps:
- Launch planning, detailing the pricing and contracting strategy, trade partner and channel blueprinting, and order-to-cash process development are principal at this stage.
- Instituting the commercial accounting function and process ideally begins 12 months pre-approval, including defined processes to account for commercial contracts, integrate order-to-cash with third party providers, account and track inventory and perform contract profitability analytics.
- Lastly, closer to launch (12-6 months out), companies should build-out their gross-to-net (GTN) process and controls, with models/methodologies for GTN accruals, a governance structure and revenue management capabilities.
Product launch is incredibly exciting! But, with it comes accelerated maturity needs. Organizations that plan proactively and strategically reduce manual and risk-laden tasks will be better positioned for commercial success! Leaning into software for clinical trial forecasting and accruals has proven to be a strategic 'win' for today's biotech organizations as they scale-up for commercial revenue readiness.
Get in touch with our team to learn more
Share this
- November 2024 (1)
- October 2024 (2)
- September 2024 (1)
- August 2024 (6)
- June 2024 (2)
- December 2023 (1)
- October 2023 (1)
- September 2023 (4)
- April 2023 (1)
- February 2023 (2)
- January 2023 (2)
- December 2022 (1)
- November 2022 (2)
- October 2022 (3)
- September 2022 (4)
- August 2022 (2)
- July 2022 (2)
- June 2022 (2)
- May 2022 (3)
- April 2022 (3)
- March 2022 (1)
- August 2021 (1)
